Labour is a dynamic process, with each birthing person experiencing the pain of labour differently. Effective pain management involves understanding the range of available strategies and choosing the options that best support comfort, control, confidence, and enhance coping throughout each stage of labour. This infographic outlines both non-pharmacologic and pharmacologic pain management techniques—highlighting practical, evidence-informed methods that can be used alone or in combination. By exploring these approaches, labouring individuals, care providers, and support persons can make informed decisions that align with personal preferences, clinical needs, and support the progression of labour.
Clinical Practice Resources
|
|
|
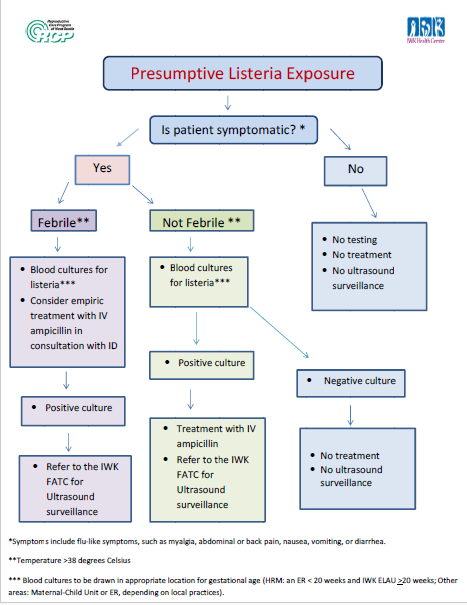
In response to recent questions about exposure to products that may have been contaminated with listeria, we would like to remind providers of a testing and treatment algorithm that was developed by the IWK and RCP for pregnant patient with presumptive listeria exposure. The FATC referral form can be found on the IWK website. |
|
This resource has been developed by care providers at IWK Health with contribution from the Reproductive Care Program. The goal is to offer prenatal care providers across Nova Scotia with an information resource that will guide pregnant persons regarding fetal movement. |
|
This practice resource provides prenatal care providers with screening and treatment guidance for anemia in pregnancy, based on evidence as of the date of the most recent version. The algorithm distinguishes between the types of anemia that may be encountered, presents the health professional with the most appropriate treatment options, and provides recommendations for follow-up assessments. |
|
This practice resource, developed in September 2021 and revised in February 2022, provides information to care providers related to a new approach to GDM screening in Nova Scotia. The practice resource has been informed by evidence and has been adapted specifically to Nova Scotia’s context and population of pregnant persons. It includes the Diabetes Care Program of Nova Scotia GDM screening practice algorithm and information related to the interpretation of testing results. In January 2022, Central Zone Pathology and Laboratory Medicine issued a memo outlining the process for ordering HbA1C based on the new provincial approach to GDM screening. In June 2022, the Diabetes Team at the IWK issued a memo outlining operational changes in their approach to diabetes and ultrasound surveillance for diabetic patients. Additionally, you may watch New Approach to GDM Screening in Nova Scotia, a recorded presentation given by Dr. Jillian Coolen on October 25, 2021 as part of RCP's Webinar Series. Pregnancy and Diabetes: Approaches to Practice (2021): This resource, published by the Diabetes Care Program of Nova Scotia (DCPNS), provides health care providers with additional information in support of recognized Clinical Practice Guidelines for the management of pregnancy complicated by diabetes. |
|
The Nova Scotia Vaccine Expert Panel has updated resources for care providers to use with pregnant and breastfeeding individuals to guide discussion about COVID-19 vaccination. This information is specific to adult immunization. In addition to these documents, the IWK has produced a video to help individuals make an informed choice about whether to get the COVID vaccine while they are pregnant, trying to get pregnant or breastfeeding. The video is hosted on the IWK public website: https://www.iwk.nshealth.ca/COVID-19/vaccine-info-for-pregnant-or-breastfeeding For more resources related to the care of pregnant patients and their newborns affected by COVID-19, please refer to our COVID-19 Resources page. |
|
Accurate assessment of gestational age is crucial:
Consistent approaches to gestational age assessment must also be taken to optimize accuracy. This provincial guideline has been revised to reflect the recommendations of national professional organizations and local experts, within the context of Nova Scotia perinatal care provision. As such, these guidelines may differ from those found in other jurisdictions. The RCP acknowledges the Divisions of Maternal Fetal Medicine (MFM) and Neonatal Perinatal Medicine (NPM) at the IWK Health Centre, for their collaboration in producing this guideline. |
Syphilis and Screening in NS for Pregnant Persons and NewbornsThe rate of syphilis in Canada has been steadily rising in recent years, becoming a significant public health concern. A surge in syphilis infection among women of childbearing age has led to more cases of congenital syphilis (transmission from the pregnant person to the fetus during pregnancy), which can result in severe health outcomes or death in newborns. An infographic created by the Public Health Agency of Canada provides a visual aid depicting the increase in syphilis rates in Canada. Cases of syphilis in pregnancy and confirmed cases of syphilitic stillbirth are reportable to Public Health. The rise in cases has led to national research exploring strategies to address infectious and congenital syphilis in Canada. ScreeningScreening recommendations for syphilis during the antenatal and postpartum periods, and for newborns are provided below. These recommendations have been in place since 2020, were adapted from national guidelines, and remain in place until further notice. See the letter to care providers from January 2020 for additional practice guidance regarding consultation, testing and treatment.
Nova Scotia Department of Health and Wellness Syphilis Surveillance GuidelinesThe Nova Scotia Department of Health and Wellness have updated the Syphilis Surveillance Guidelines and they are now available. The update comprises the addition of new stages for congenital syphilis, including late congenital syphilis and syphilitic stillbirth. Surveillance Guidelines for congenital syphilis and non-congenital syphilis have been separated into two documents, both of which also contain guidelines for identifying syphilis outbreaks. For more information please access the Syphilis section of the Communicable Disease Manual that is accessible on the Nova Scotia Department of Health and Wellness website. Provincial Syphilis Outbreak: Recommendations for pregnant persons and newbornsAs of January 20, 2020, the Office of the Chief Medical Officer of Health for Nova Scotia has declared a provincial syphilis outbreak. Across Canada syphilis outbreaks have been declared in most provinces/territories, due to increasing rates of infection. Changes to recommendations for pregnant women and newborns are outlined in the documents below: |
|
The Canadian Paediatric Society (CPS) has recommended moving from universal newborn ocular prophylaxis, to universal prenatal screening for Neisseriae gonorrhoeae (GC) and Chlamydia trachomatis (CT) and treatment of those with positive results in order to eradicate infection and prevent intrapartum transmission to the newborn. As universal ocular prophylaxis is eliminated from routine newborn care, functions of the health system must be optimized and synchronized to prevent ON. RCP has partnered with clinical experts and stakeholders from across NS to produce these resources, which are designed to offer guidance for the prevention of ON: |
Key messages for health care providers
For more information about Lyme disease, refer to the following resources: From the Government of Canada and the Public Health Agency of Canada
From the Nova Scotia Department of Health & Wellness |
|
A detailed guide and reference for prenatal care providers using the Nova Scotia Prenatal Record to document assessment, investigation and treatment during pregnancy. Includes instructions on assembly of the prenatal record, a glossary of terms related to pregnancy and prenatal care, details on completing each section of the record, guidelines for antenatal screening and related resources. |
|
The Nova Scotia guidelines for antenatal laboratory screening and testing were revised and re-released in 2024. The guidelines are available in a convenient printable card format. The companion document to the new Nova Scotia Prenatal Record provides detailed information on applying these guidelines. |